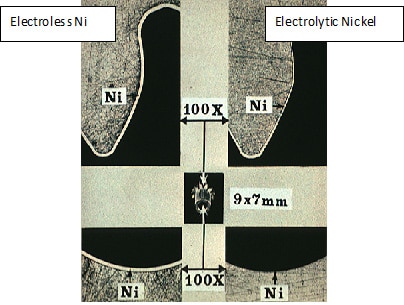
First developed in the 1940s electroless nickel en plating is used heavily by automotive and aviation for corrosion and wear resistance.
Electroless nickel plating electrical conductivity.
The thermal conductivity of an electroless nickel deposit containing 8 to 9 percent phosphorus is 0 0105 to 0 0135 cal cm sec c.
This plating will provide corrosion resistance and maintain electrical conductivity while reducing reflectivity on the surface.
They are conductive but not as much as some other metals.
Heat treatments precipitate phosphorus from the alloy and can increase the conductivity of electroless nickel by 2 to 4 times.
For example 3 p gives a resistance of about 30 micro ohm cm 8 9 p gives a resistance of about 90 110 micro ohm cm.
Not only are the electrical properties of gold plating desirable but gold plating results in the following other desirable properties.
Black nickel is electrolytic nickel plating with a secondary treatment to turn the surface black.
Electrodeposited nickel has a value of 0 19 to 0 26 cal cm sec c.
Electrical thermal conductivity the thermal and electrical properties of electroless nickel coatings will vary with phosphorus content.
The formulation of the plating solution can also affect conductivity.
Excellent corrosion resistance excellent solderability ability to withstand high temperatures and superior wear.
Gold plating is applied to provide excellent electrical conductivity for many industries.
It is a coating with multi functional properties.
Conductive textiles coated with nickel aluminum silver and copper are important types of materials for emi shielding.
Hardness heat hardenability solderability electrical conductivity and abrasion wear and corrosion resistance.
Nickel electroplating electroplating or surface treatment involves applying a thin layer of metal or metal alloy.
There is a difference in electrical conductivity properties.
The electrical resistivity and thermal conductivity of high phosphorus en is generally 50 200 micro ohms cm and 0 08 w cm2 k respectively.
Hard chrome and electroless nickel plating aren t typically used for their electrical conductivity attributes.
The specific resistance of electroless nickel with 6 7 p is about 50 70 micro ohm cm in general the more phosphorus the more resistance.
The electrical conductivity of nickel is 9 7 10 4 s cm 7 9 26.
Tests with baths complexed with sodium acetate and with succinic acid showed electrical resistivities of 61 and 804μω cm respectively.